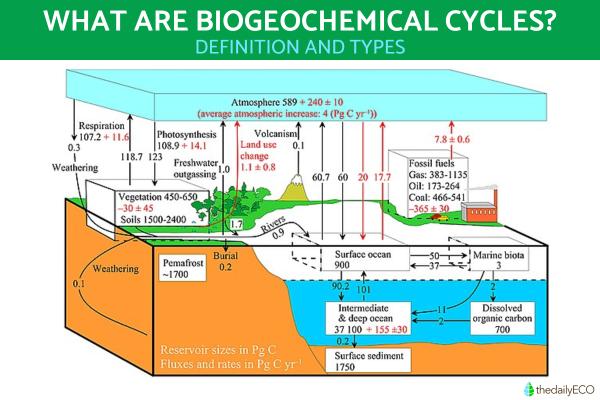
Considered cycles of matter, biogeochemical cycles are the natural routes of transformation by which essential elements and compounds move between living organisms, the atmosphere, the Earth's crust and water. These cycles are continuous and are necessary for the regulation of the nutrients essential for life on Earth. Since they are cycles, they exhibit a closed movement which allows these elements and compounds to be recycled, unlike the open flow of energy in ecosystems.
Understanding the characteristics and types of biogeochemical cycles helps us understand ecosystem dynamics. In doing so, we can also discover how human activities can alter them and what threat doing so poses to our ecosystems. We learn more as thedailyECO asks what are biogeochemical cycles?
What are biogeochemical cycles?
Biogeochemical cycles are the processes that constantly recycle the elements and chemical compounds that are necessary for life on Earth. These cycles occur between living organisms, the Earth's crust, the atmosphere and water. Essentially, these cycles exist between the biological, geological and chemical components of our planet. Living organisms use biogeochemical cycles to obtain nutrients and they are necessary to maintain ecological balance.
In these natural cycles of matter, macronutrients and micronutrients that constitute the inorganic matter present in our environment (air, water or soil) are incorporated into organisms as organic matter. This occurs through metabolic processes after which they subsequently return to the natural environment in their inorganic form.
Macronutrients (CHNOPS) constitute more than 95% of the biomass of all living beings. These are the elements that our body requires in large quantities for its development, maintenance and reproduction. Although they are also essential, micronutrients are less prevalent in the body than macronutrients. Some examples of micronutrients in biogeochemical cycles are iron (Fe), copper (Cu), zinc (Zn), chlorine (Cl) and iodine (I).
Types of biogeochemical cycles
There are different ways we can categorize the different types of biogeochemical cycles. These include factors such as complexity, mobility and location. We look at these categorizations of matter cycles below:
Types of biogeochemical cycles according to complexity
- Simple cycles: where the elements are influenced more by physical-chemical forces than by biological ones. Examples include salts and trace elements.
- Intermediate cycles: made up of elements of organic matter (OM) that can be easily released (CHOP).
- Complex cycles: associated with elements of organic matter that require specialized microorganisms in their complex transformations (N and S).
Types of biogeochemical cycles according to mobility
- Global cycles: these are biogeochemical cycles that have gaseous phases, something which allows their distribution on a global scale.
- Local cycles: these are less mobile and more sedimentary cycles. They end up being transported by water and accumulating in sediments, giving rise to a more regional or local distribution (e.g., P, K and Ca).
Types of biogeochemical cycles according to location
- Gaseous: macro- and micronutrients are rapidly recycled and circulate between the atmosphere and living beings. These include the oxygen, carbon and nitrogen cycles.
- Sedimentary: elements such as phosphorus and sulfur circulate between the Earth's crust, hydrosphere and living organisms. They are recycled at a slower rate than those in the gaseous cycle.
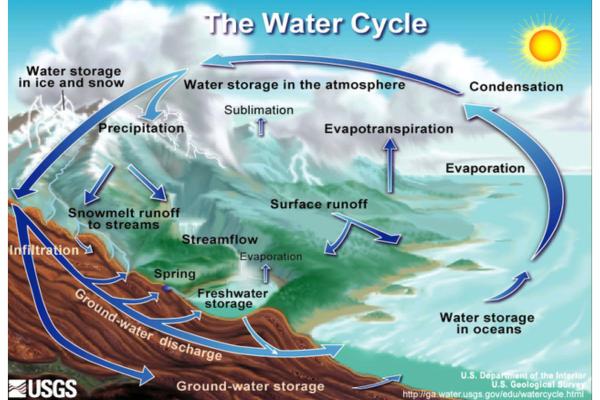
- Hydrological: take part in major reservoirs such as rivers, oceans, lakes and even groundwater. The elements and compounds are dissolved and transported through the water itself.
You can learn more about this hydrological recycling of elements and compounds with our article asking what is the water cycle and its importance?
Carbon cycle
The carbon cycle is essential because it shapes organic matter and represents the exchanges between living organisms and the environment. It is a result of the processes of respiration and photosynthesis.
Carbon is generally recycled rapidly, although it can remain in unavailable forms for long periods. In warm and humid ecosystems such as tropical rainforests, production and decomposition rates are high. This allows carbon to circulate rapidly through the ecosystem. In contrast, the process is slower in cold and dry ecosystems. This is one of the reasons why rainforests can act as carbon sinks which keeps the carbon from entering the atmosphere[1].
While this provides a basic understanding of this type of biogeochemical cycle, you can learn more with our article on understanding the carbon cycle.
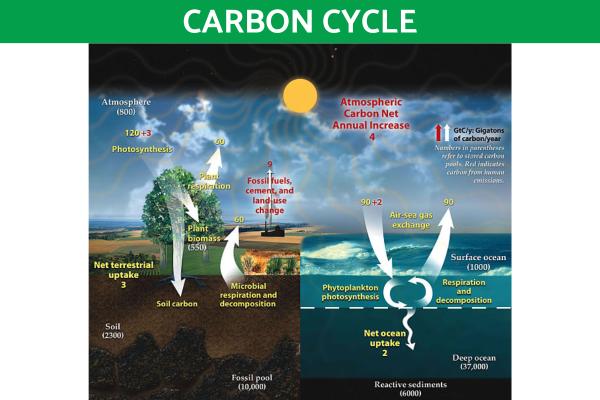
Sulfur cycle
The chemical element sulfur has both sedimentary and gaseous phases, as well as a hydrological phase. This affects the nature of this type of biogeochemical cycle:
- Sedimentary sulfur cycle phase: sulfur is immobilized in organic and inorganic deposits. It is then released through weathering and decomposition processes until it is transported to terrestrial ecosystems in the form of saline solution.
- Gaseous sulfur cycle phase: composed of sulfur dioxide (SO2) and hydrogen sulfide (H2S) which allows the circulation of sulfur on a global scale.
- Hydrological sulfur phase: sulfur dissolves in lakes, rivers and oceans in the form of sulfate ions (SO₄²⁻), being used by various aquatic organisms for various biological processes. It can move from atmospheric gaseous phases when falling as acid rain.
Volcanic eruptions are important aspects of the sulfur cycle, since they released an incredible amount of this element into the atmosphere. You can see these various phases of the sulfur cycle in the diagram below and you can read our article on what causes a volcano to erupt to learn more.
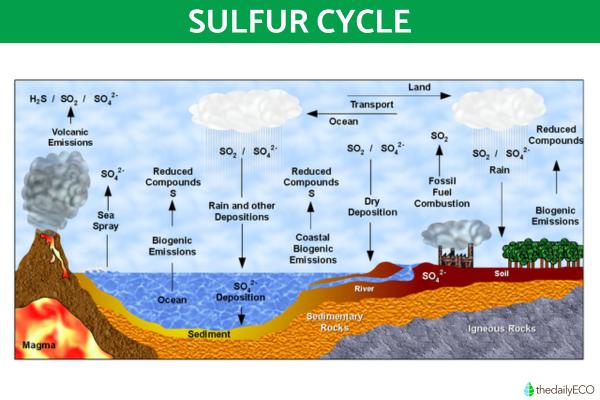
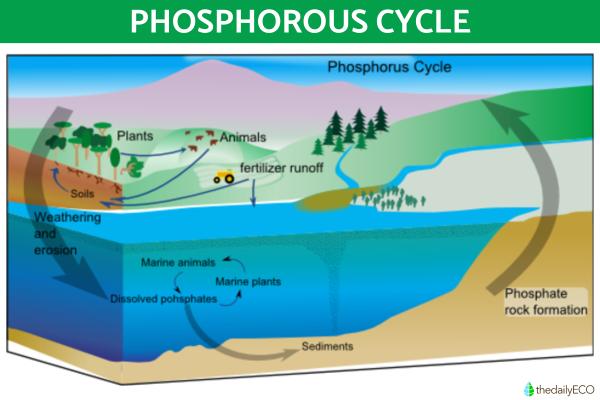
Phosphorus cycle
The biogeochemical cycle of phosphorus does not have a significant atmospheric reservoir, as it is found mainly in mineral deposits and marine sediments in unavailable forms. It is released into terrestrial and aquatic ecosystems mainly through rock erosion and mining extraction. Living organisms need phosphorous as it is essential for the creation DNA, RNA and other building blocks of cells.
You can see this phosphorous cycle demonstrated in the diagram below:
Why are biogeochemical cycles so important?
The importance of biogeochemical cycles is due to the benefits they provide both living organisms and inorganic material, as well as how their characteristics influence global ecosystems:
- Facilitate life: first and foremost, these cycles enable life on Earth by maintaining optimal conditions. This means that biogeochemical cycles regulate the climate, nutrient distribution and other vital processes.
- Matter exchange: they also enable the exchange of matter between living beings and the natural environment and access to the vital elements (nutrients) we need.
Learn more about the beginnings of our planet with our article asking how was Earth originally formed?
What is the human impact on biogeochemical cycles?
Humans are living organisms which benefit from biogeochemical reactions and without which we would die. Despite our reliance on the continued functioning of these cycles of matter, human activity is having serious deleterious effects on them. They include:
- Deforestation: the effects of deforestation include alterations the water cycle, leading to the desertification of ecosystems.
- Pollution: wastewater discharges, intensive agriculture and the use of fertilizers (eutrophication) modify the nitrogen and sulfur cycles, promoting acid rain in the process. You can learn about the types of water pollutants in our related guide.
- Overfishing: large-scale fishing activities alter bacterioplankton, potentially modifying the C, N, O and P cycles it regulates.
- Climate change: industrial activities and the burning of fossil fuels modify, among others (such as S), the carbon cycle, causing global warming.
To learn more about these effects of changes to biogeochemical cycles, take a look at our article explaining the difference between climate change and global warming.
If you want to read similar articles to What Are Biogeochemical Cycles?, we recommend you visit our Biology category.
1. Heinrich, V. H. A., Vancutsem, C., Dalagnol, R., Rosan, T. M., Fawcett, D., Silva-Junior, C. H. L., Cassol, H. L. G., Achard, F., Jucker, T., Silva, C. A., House, J., Sitch, S., Hales, T. C., & Aragão, L. E. O. C. (2023). The carbon sink of secondary and degraded humid tropical forests. Nature, 615(7952), 436–442.
https://doi.org/10.1038/s41586-022-05679-w